The N6-methyladenosine (m6A)-dependent gene regulation has emerged as a prevalent and selective post-transcriptional modification that profoundly influences cell fate by modulating RNA splicing, translation and stability. This modification is orchestrated by a core methyltransferase complex composed of METTL3, METTL14 and WTAP; reversed by the demethylases, including FTO and ALKBH5; and interpreted by reader proteins, such as the YTH domain-containing family members (YTHDF1/2/3) and IGF2 mRNA binding proteins (IGF2BP1/2/3). m6A modification mediates various immune regulations, including T cell survival by targeting Orai1 and Ripk1, and T cell infiltration within the acidic tumor microenvironment (TME) via targeting ITGB1.
Moreover, m6A modification in tumor cells can regulate immune responses to anti-PD-1 immunotherapy. However, the specific m6A-regulated target genes that control anti-tumor immunity within T cells remain poorly understood.
Programmed cell death protein 1 (PD-1) is a cell surface immune checkpoint that regulates the adaptive immune response, and facilitating tumor immune evasion (TIE). Therapeutic antibodies targeting PD-1 or its ligand PD-L1 have become the standard-of-care treatments, significantly improving overall survival across multiple cancer types. However, a substantial proportion of patients derive limited clinical benefit, underscoring the need for a more comprehensive understanding of PD-1 regulation. To date, most studies on PDCD1 regulation have focused on the transcriptional and post-translational mechanisms. However, it remains unclear whether PDCD1 is subject to post-transcriptional regulation, such as through m6A.
Programmed cell death protein 1 (PD-1) is a cell surface immune checkpoint that regulates the adaptive immune response, and facilitating tumor immune evasion (TIE). Therapeutic antibodies targeting PD-1 or its ligand PD-L1 have become the standard-of-care treatments, significantly improving overall survival across multiple cancer types.However, a substantial proportion of patients derive limited clinical benefit, underscoring the need for a more comprehensive understanding of PD-1 regulation. To date, most studies on PDCD1 regulation have focused on the transcriptional and post-translational mechanisms. However, it remains unclear whether PDCD1 is subject to post-transcriptional regulation, such as through m6A.
Recently, a team led by Professor Shan Gao published a research paper titled METTL14 Enhances Anti-Tumor Immunity through m6A-dependent loss of PD-1 online in Cancer Research, providing a new theoretical basis and therapeutic strategy for improving cancer immunotherapy.

Based on analysis of public databases, researchers confirmed that m6A modifications are enriched in both the coding sequence (CDS) and the 3' untranslated region (3'UTR) of the PDCD1 gene. Further studies revealed that the methyltransferase complex component METTL14 and the demethylase ALKBH5 collaboratively regulate the m6A modification level of PDCD1, as well as its mRNA and protein expression. Meanwhile, the reader proteins YTHDF1/2/3 mediate the degradation of PDCD1 mRNA in an m6A-dependent manner. Using a T cell-specific gene knockout mouse model, the molecular mechanism by which METTL14 regulates PD-1 expression via m6A modification was validated in vivo.
The study found that loss of METTL14 leads to impaired CD8⁺ T cell function and accelerated tumor growth. Clinical data analysis showed that METTL14 expression is negatively correlated with PDCD1 levels and is closely associated with resistance to PD-1 immunotherapy. Importantly, the research team employed the METTL3–METTL14 complex degrader WD6305 in combination with an anti–PD-1 antibody, which exhibited a significant synergistic antitumor effect. This discovery provides an innovative combination strategy to overcome resistance to tumor immunotherapy and holds considerable promise for clinical application.
In summary, this work reveals an m6A-dependent regulatory mechanism for PDCD1 in T cells and demonstrates the critical role of the METTL14–PD-1 axis in anti-tumor immunity. These findings offer novel perspectives for developing T cell-targeted immunotherapeutic strategies.
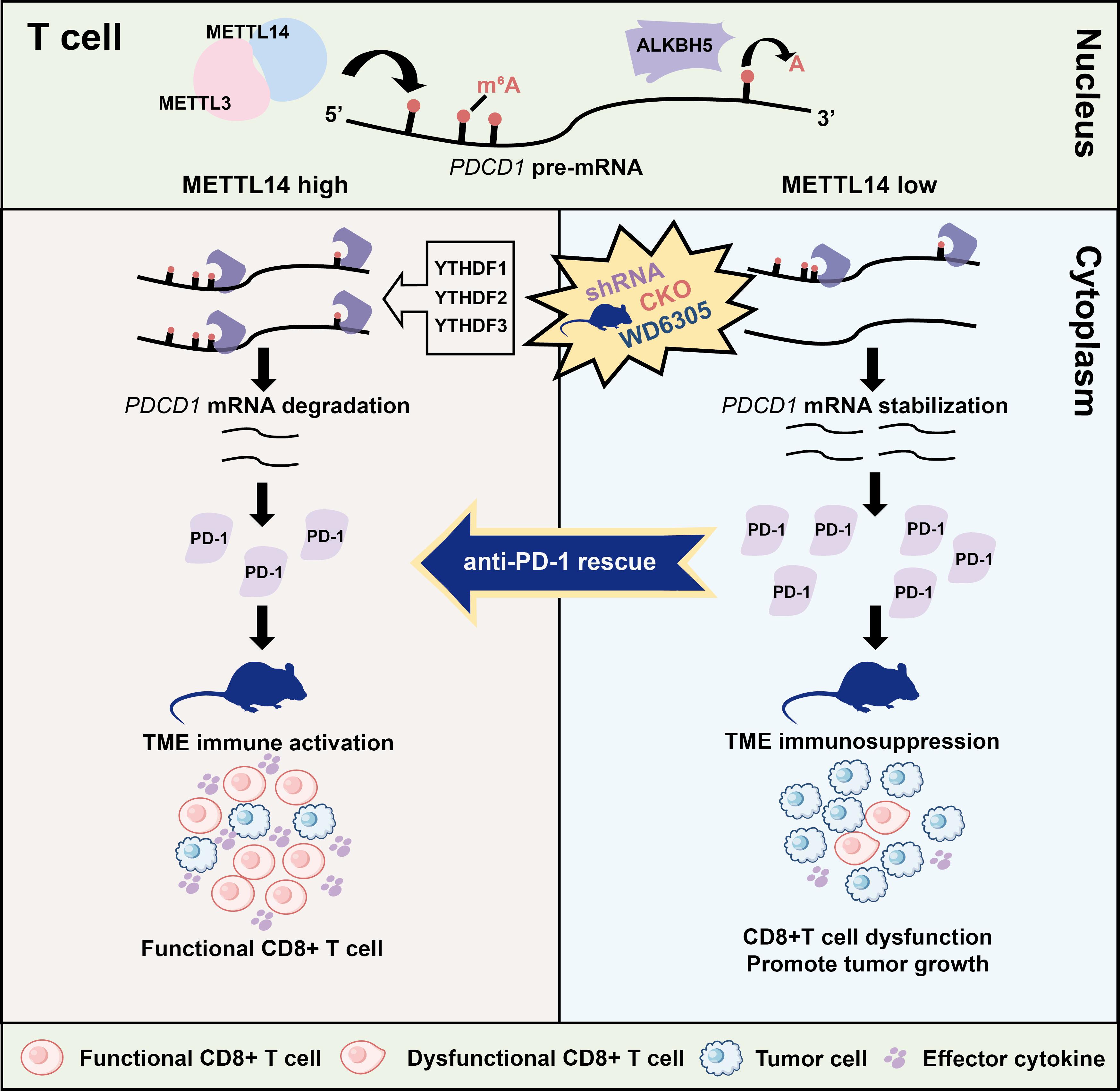
Proposed model for the METTL14-YTHDF1/2/3-PD-1 axis in T cells regulates anti-tumor immunity
Huang Chang, a co-supervised Ph.D. candidate from Southeast University and Guizhou University, is the first author of the paper, and Professor Shan Gao is the lead corresponding author.
Link to the paper:https://doi.org/10.1158/0008-5472.CAN-25-0065

